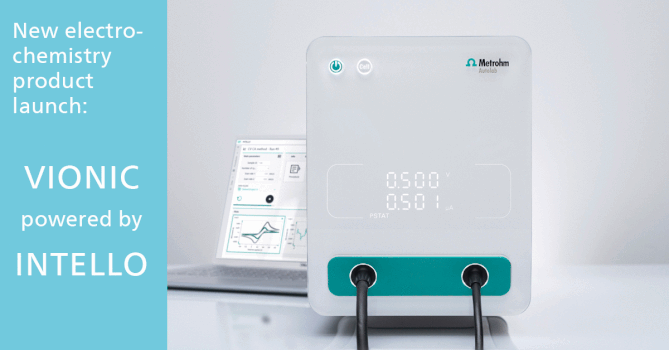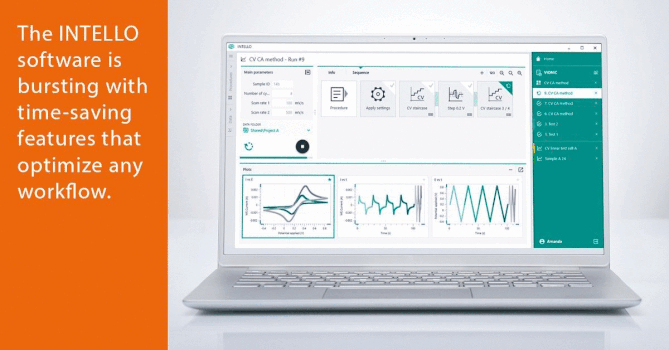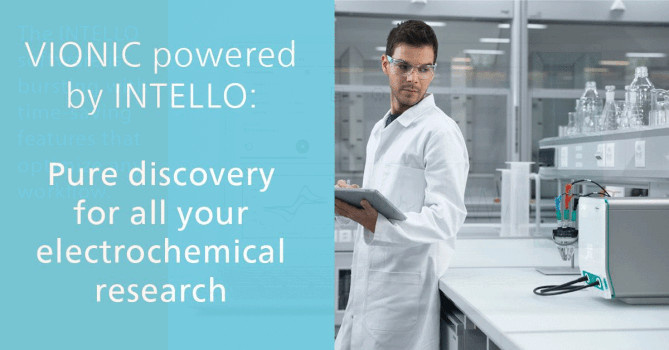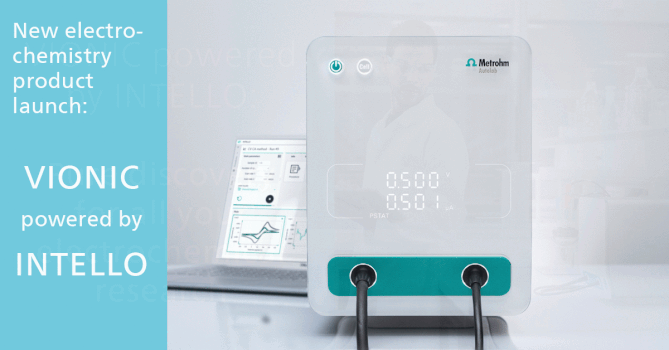
The Basics of Gas-Phase Ion Chemistry
Keywords:
proton affinity, gas-phase basicity, reaction coordinate, kinetics, rearrangements vs. simple bond cleavage, kinetic shift, ion structure and energy, reactivity, transition statesAbstract
This article deals with the fundamental definitions used in mass spectrometry and gas-phase ion chemistry. Positive ions are characterized by ionization energy, proton affinity and gas-phase basicity. Negative ions are characterized by electron affinity and gas-phase acidity. Also discussed are the fragmentation energetics and types of potential energy diagrams. Kinetic aspects of ion dissociations are discussed with respect to charge distribution in even-electron and odd-electron ions, and parameters controlling rate constants, such as activation energy and frequency factors.