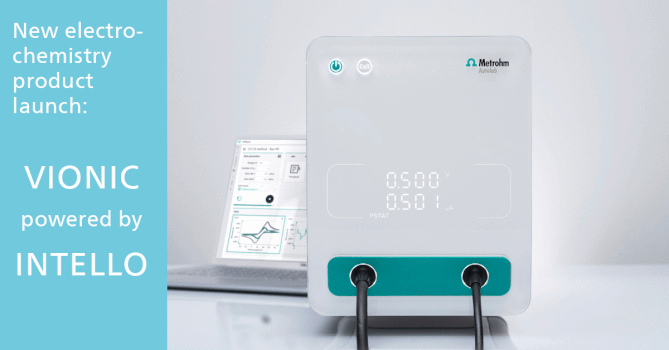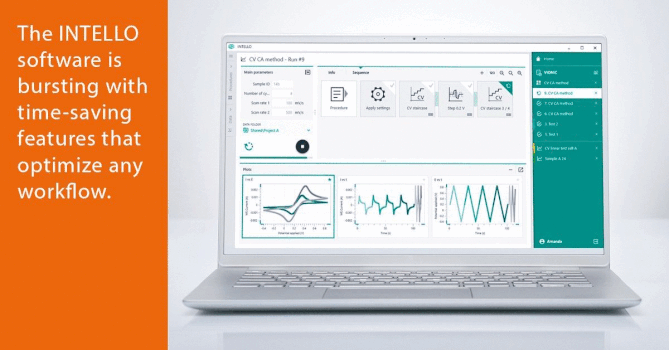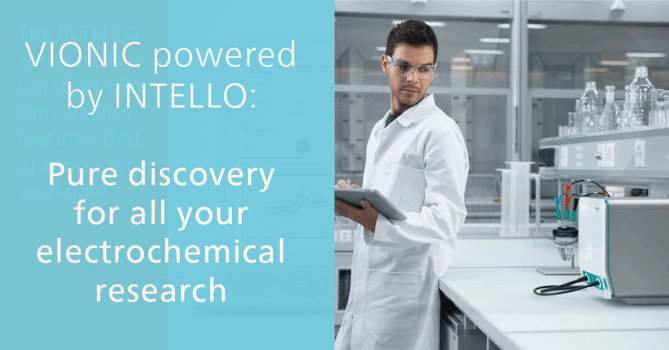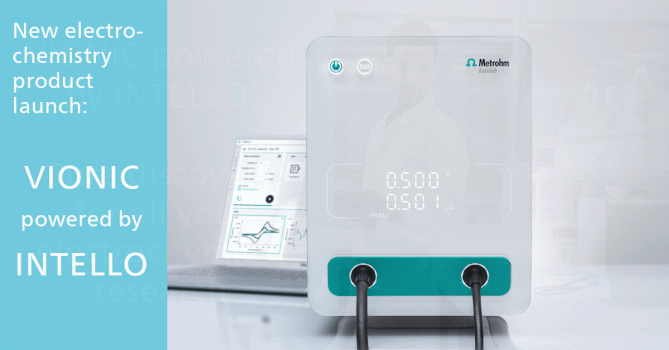
Validation of Production of Single-dose Medicated Nanofiber Matrices Manufactured by Electrospinning
Keywords:
weight uniformity, content uniformity, rizatriptan, nanotechnologyAbstract
A considerable obstacle of the real nanofiber use in drug dosage forms consists in the low weight of the doses and in the content non-uniformity of medicated nanofiber products, especially of those manufactured on a large scale. The electrospinning nanofiber technology is the most promising in this context; however, no results obtained by validated production have been published so far. Here we present the results of the validation study of electrospun medicated strips intended for administration of rizatriptan as a drug medicated in milligrams per dose. The results obtained demonstrate a compliance with the good manufacturing practice and pharmacopoeial requirements on weight and content uniformity. We have concluded that the tested Nanospider™ electrospinning technology is validable and thus suitable for a wide number of drugs intended for single-dose administration of small drug quantities.
English translation available in the on-line version.