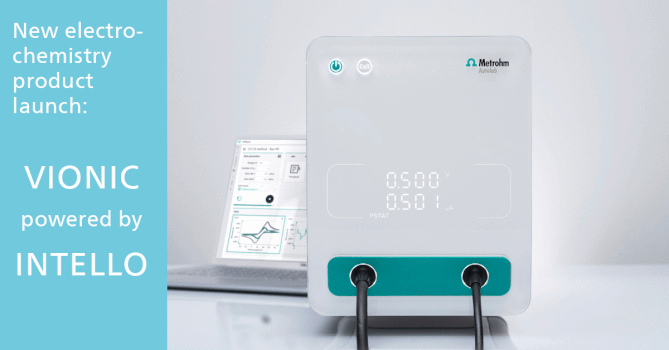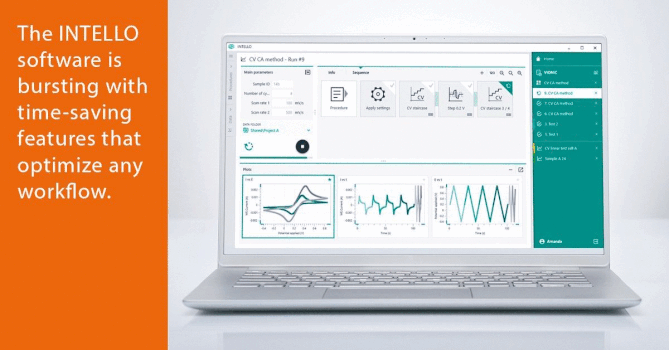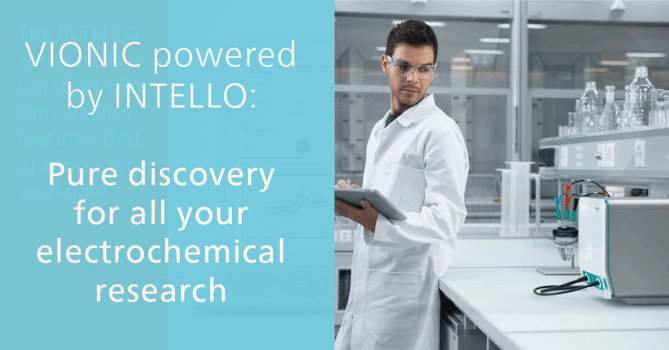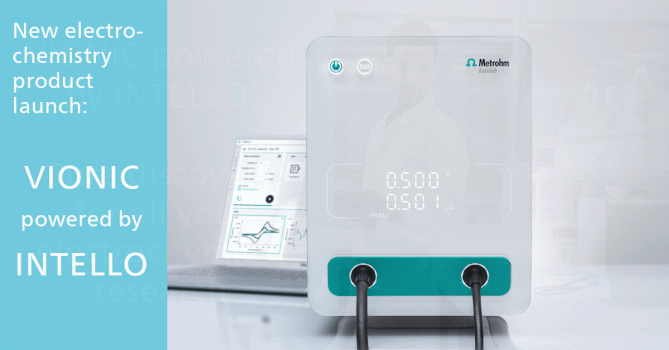
Role of Water in Chemistry: Molecular Point of View
Keywords:
solvent, nanoparticles, clusters, molecular beams, mass spectrometryAbstract
Water is the most important solvent in chemical reactions. However, its role does not have to be always obvious or correspond to our chemical intuition. In the present short review article, we focus on the role of water at the lowest, molecular level. We also briefly discuss the methods being used to study the hydrated molecules and ions. We show that already a few water molecules can entirely change the course of atmospheric reactions, protect DNA building blocks from dissociation or create various isomers of a hydrated electron in clusters with metal ions.