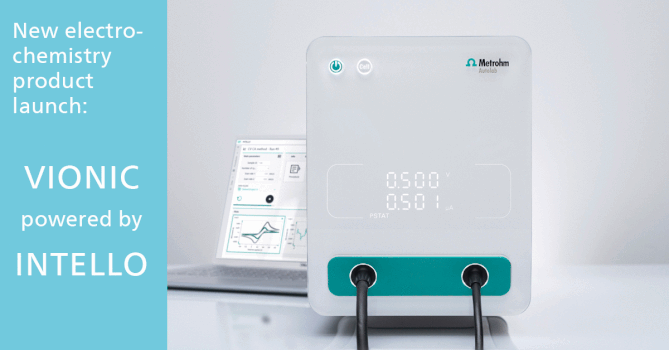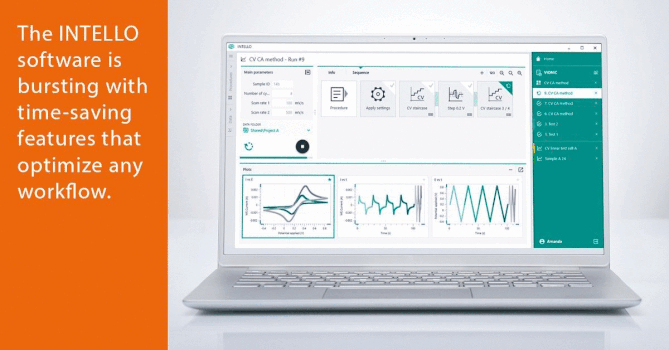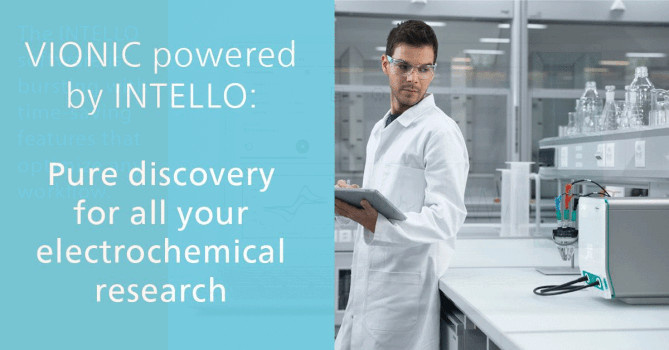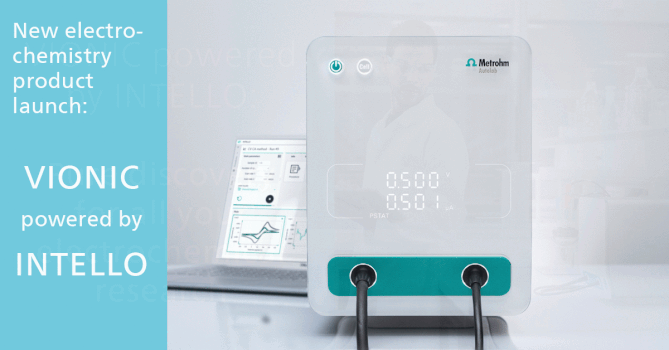
Data Integrity in Pharmaceutical Industry
Keywords:
data integrity, pharmaceutical industry, quality control, GMP/cGMP, GAMPAbstract
Data integrity requirements in the pharmaceutical industry are more important and more urgent than ever before. This is due to the development, incessant changes and the tightening of the legislative requirements for the critical steps throughout the pharmaceutical product life cycle from research to commercial production, and must be accessible for further use. In addition, the data must be traceable and continuous in any pharmaceutical company during various phases of the product life cycle. The pharmaceutical industry should strategically consider the approach to the data integrity of computer systems related to Good Manufacturing Practice in order to meet the respective requirements of regulatory agencies.